83.甲泼尼龙
出处:按学科分类—医药、卫生 军事医学科学出版社《临床常用进口药物手册》第458页(31375字)
【中文释文】:
甲泼尼龙琥珀酸钠
〔性状〕
甲泼尼龙(甲基强的松龙)琥珀酸钠外观为白色或接近白色,无味的吸湿性非晶形固体。它易溶于水和醇,不溶于氯仿,很轻微地溶于丙酮。
甲泼尼龙琥珀酸钠是泼尼松的6-甲基衍生物,极易溶于水,所以以小容量稀释剂就可以给药,而且在要求迅速出现高血药浓度的甲泼尼龙琥珀酸钠的情况下,特别适于静脉使用。甲泼尼龙(甲泼尼龙琥珀酸钠)有以下几种溶液:
40mg混合小瓶:每毫升(混合时)含甲泼尼龙琥珀酸钠相当于40mg甲泼尼龙;同时含有二磷酸钠;干燥磷酸钠乳糖;苯甲醇和注射用水。
125mg混合小瓶;每瓶2ml(当混合时)含甲泼尼龙琥珀酸钠相当于125mg甲泼尼龙;同时含有二磷酸钠;干燥磷酸钠;苯甲醇和注射用水。
500mg小瓶:每瓶8ml(按规定混合时)含甲泼尼龙琥珀酸钠相当于500mg甲泼尼龙,同时含有二磷酸钠,干燥磷酸钠,苯甲醇和注射用水。
1000mg小瓶:每瓶16ml(当按规定混合时)含甲泼尼龙琥珀酸钠相当于1000mg甲泼尼龙,同时含二磷酸钠;干燥磷酸钠、苯甲醇和注射用水。
2g小瓶:每瓶(按规定混合时)甲泼尼龙琥珀酸钠相当于2g甲泼尼龙,同时含有二磷酸钠,干燥磷酸钠,苯甲醇和注射用水。
必要时,125mg、500mg、1000mg和2g处方可以用氢氧化钠和(或)盐酸调pH。注意:溶解玻璃瓶中甲泼尼龙时,只能使用附带的稀释剂。
〔作用〕
甲泼尼龙是普强公司研究室合成的强力抗炎甾体化合物。它比泼尼松龙有更强的抗炎作用。比泼尼松龙导致钠潴留、水潴留的倾向性更小。
甲泼尼龙琥珀酸钠像甲泼尼龙一样,有同样的代谢和抗炎作用。肠胃外等克分子给药时,两种化合物有相等的生物活性。甲泼尼龙(甲泼尼龙琥珀酸钠)和氢化可的松琥珀酸钠静脉给药后,以嗜酸性细胞数的阻抑为指标,其药效比至少是4∶1。这与甲泼尼龙和氢化可的松口服液比较药效是完全一致的。
〔适应证〕
在需要迅速、强力激素作用情况下,适于静脉给药,包括:
1.内分泌失调急性肾上腺皮质功能不足(氢化可的松或可的松是可供选择药物;盐肾上腺皮质素类的供给也可能是必要的,特别是使用同类合成药物时)。
胶原性疾病,在选择的系统性红斑狼疮病例中病情加剧或维持性治疗期间。
2.皮肤病
(1)天疱疮。
(2)严重多形(性)红斑(斯-约二氏综合征)。
(3)鳞片样脱发症(剥脱性皮炎)。
(4)扩散性神经炎。
3.过敏性状态 控制用常规治疗的适当疗法不能奏效的严重的或不能过敏的状态。
(1)支气管哮喘。
(2)接触性皮炎。
(3)血清疾病。
(4)药物过敏反应。
(5)用于控制严重的或不能过敏的状态;用适当的常规治疗难以奏效的血管神经性水肿、荨麻疹以及过敏时肾上腺的辅助药物。
4.胃肠疾病 使溃疡性结肠炎病人度过危象期(系统治疗或灌肠或滴注)。
5.大脑水肿 大脑水肿使用皮质甾类药物旨在减少或预防由脑瘤(原发性或转移性)、脑外伤和颅内手术引起的大脑水肿。
对于由肿瘤引起水肿的病人,为了避免颅内压回跳性增加,逐渐减少肾上腺皮质激素类剂量似乎是重要的。如果随着剂量减小发生脑水肿(颅内出血被排除),要重新开始肠胃外给药使用较大剂量,增加用药次数。患有某些恶性病的病人可能需要继续口服肾上腺皮质激素治疗(例如,12~36mg/d甲泼尼龙)数月甚至终生。相同剂量或更高剂量可能有助于控制放射治疗期间水肿。脑外伤引起的水肿应尽可能早期治疗。
1.器官移植 预防或治疗对器官移植的排斥反应。
2.急性风湿热
3.严重休克 出血性,外伤,手术休克。
辅助静脉使用溶剂的甲泼尼龙可能达到血流动力学恢复。皮质激素疗法不应代替治疗休克的常规方法,但有证据表明,同时使用大剂量皮质激素和其他措施可提高存活率。
1.做为治疗哮喘的辅助药物 一些研究人员发现,婴儿和儿童作用皮质激素结合采用其他可接受的治疗措施,在治疗中度重症和严重哮喘(急性气管炎)时是有价值的。然而,目前还没有关于皮质激素治疗由马流感引起的会厌型哮喘效果的资料,没有使用甲泼尼龙可减少气管造口术的证据。规定剂量40mg,肌肉注射,发作时尽早给药。这种疗法耐受性良好。据报道,用该药治疗偶尔可发生短暂性心动徐缓。
2.食管烧伤 由于摄入腐蚀剂而引起食管烧伤,使用皮质甾类药可降低食管狭窄形成的发生率并降低发病率。为了使药物有疗效,烧伤后48h内必须开始使用皮质激素。作用迅速的甾类药物,例如甲泼尼龙琥珀酸钠可以同支持液和抗生素结合给药做为最初的治疗。食管镜检后,确定无烧伤的病人可停止使用该药。食管受损的病人应继续用聚解-甲泼尼龙(醋酸甲泼尼龙)或口服甲泼尼龙。如果耐受良好,需要部位可用抗生素和探条扩张术。
〔禁忌证〕
除在过敏反应时用于短期或急救治疗外,甲泼尼龙琥珀酸钠像其他任何皮质激素药物一样,患有单纯性角膜炎、急性精神病和潜在的已愈合或活动性结核病的病人应绝对禁用。然而,同时使用皮质甾类药物和抗结核制剂可能挽救某些肺结核或脑膜结核病人的生命。除非已经表明,结核杆菌对正在使用的抗结核制剂敏感,不应使用皮质甾类药物。下列情况应认为与禁忌有关:活动性或潜在性消化性溃疡、柯兴综合征(肾上腺皮质功能亢进)、憩室炎、肠吻合术后、骨质疏松、肾功能不全、血栓栓塞倾向、精神病倾向、糖尿病、高血压、局部或全身性感染,包括牛痘、水痘以及霉菌病和其他发疹性疾病。
妊娠与皮质激素治疗的禁忌证有关,特别是前3个月,因为在实验动物身上观察到胎儿异常。如果妊娠期有必要给予皮质甾类药物,应密切观察胎儿的肾上腺功能减退征兆,如果存在这类征兆,应着手适当治疗。
如果上述情况下使用皮质激素类药物,应权衡利弊。
〔警告〕
皮质甾类药物可能引起血压升高,盐和水潴留,增加钾和钙的排泄。与可的松和氢化可的松比较,合成的皮质甾类药中的盐皮质激素类药物程度较轻。饮食限盐并补充钾可能是必要的。
用皮质激素治疗脑外伤的大脑水肿时,可能发生胃肠出血,所以每天规定做粪便愈创木脂试验对诊断是有帮助的。此外,皮质激素治疗存在可能掩盖颅内出血的可能性。
旨在确定治疗脓毒性休克使用甲泼尼龙琥珀酸钠疗效的临床研究资料表明,有血清肌酐浓度升高的病人以及开始治疗后继发性感染的病人中死亡率较高。
〔注意事项〕
只有在全面了解肾上腺皮质激素特有的活性和对肾上腺皮质激素的各种反应后才给予甲泼尼龙(甲泼尼龙琥珀酸钠)。已有几份关于接受器官移植大剂量快速静脉注射甲泼尼龙琥珀酸钠(0.5g以上)造成心血管虚脱的报道。其原因和与其他药物关系(例如,利尿剂)至今尚不清楚,但医师应警惕这种可能性。因为甲泼尼龙对纤维组织形成有抑制作用,所以可能掩盖感染体征并引起感染微生物的扩散。因此,要观察所有接受甲泼尼龙的病人是否发生中间介入感染。如发生感染,必须用适当的抗菌措施使感染得到控制。
如果可能,应避免突然停止用皮质甾类药治疗,因为感染过程迭合的肾上腺类皮质激素缺乏会造成危险。
长期用激素治疗通常会致肾上腺皮质活性和大小降低。停止治疗时相对的肾上腺皮质缺乏可以通过逐渐减少剂量加以避免。然而,潜在的极度缺乏可能无症状地延续一些时间,甚至在逐渐停止用肾上腺甾类药物后。因此,如果在治疗时或治疗已经结束一年内(偶尔也长达二年)。病人经受明显的应激,如手术、外伤或严重疾病时,在应激期间或在应激后立即增加或重新开始继续用激素治疗。由于盐皮质激素分泌可能受损,盐和(或)脱氧皮质(甾)酮应结合使用。术前和术后期间最好使用可溶激素制剂。
虽然除使用大剂量外很少遇到阻滞伤口愈合,但手术同时给予甲泼尼龙琥珀酸钠时应考虑这种情况,然而,不像甲泼尼龙琥珀酸钠那样,皮质激素类药物的平均剂量和大剂量可能引起血压升高,引起盐和水潴留以及钾、钙排出的增加。可能有必要饮食限盐和补充钾。像其他糖皮质激素一样,甲泼尼龙琥珀酸钠可能使糖尿病加重,结果可能给予大剂量胰岛素或促使潜在性糖尿病突然发生。用甾体药物治疗重症肌无力可能使其症状加重,因此必须适当注意。
在系统使用任何抗炎甾体类药物后随意肌无力已有报道。有一些病例中,这归因于低血钾。钾的过多损失,像钠的潴留一样不可能是由维持剂量的甲泼尼龙引起。然而,应该把这种作用记在心中并对长期接受任何一种甾体类药的病人进行血清钾的定期测定。
目前的研究表明,接受甾体药物治疗的病人的随意肌无力在血清钾浓度正常时可能发生,可能由于肌肉代谢紊乱而发生。发生严重肌病的病人长期以来一直接受大剂量甾类药物治疗。目前可以得到资料表明,使甾体药物治疗引起的严重肌病更经常发生在接受含9-α-氟基构型的甾体药物治疗的病人身上,在一些病例中,停止使用氟化甾体并且开始用可的松、氢化可的松或泼尼松等-甾类药物,例如,甲泼尼龙后已经看到甾体药物诱发的肌病有了改善。接受甲泼尼龙的病人,肌无力或消瘦发生率似乎较低。
接受6个月或6个月以上皮质激素类药物治疗的儿童可发生线性生长迟缓,迟缓程度大约与剂量成正比。停止治疗后,生长速度可能加速。出于这个原因,长期接受甾体药物治疗的儿童的成长应予密切注意。如果生长受到阻抑应把剂量减少到足以在骺闭合前能够恢复。胃肠外给予甲泼尼龙琥珀酸钠后发生过敏性样反应的报道非常罕见。使用该药的医师应准备应付这样的可能性。
由于某些疾病在妊娠期可自发性缓解,如风湿关节炎,所以,应尽一切努力避免妊娠期激素治疗,长期用肾上腺皮质甾类药引起胃酸过多或消化性溃疡增加,因此特别建议采取预防措施。遵守溃疡病生活方式并给予抗酸剂。主诉有胃部不适的溃疡病人应进行X线检查,无论发现变化与否,建议遵守溃疡病人生活方式。应避免在三角肌注射,因为皮下萎缩发病率很高。停用甲泼尼龙琥珀酸钠后继续监督病人是必要的,因为可能突然再现病人曾经医治过的疾病。
在治疗由脑外伤引起的脑水肿时应考虑预防消化性溃疡(如抗酸剂等)。
〔副作用〕
与使用皮质激素相关的副作用包括:柯兴综合征、满月脸、锁骨下脂肪垫、多毛症、纹和痤疮、相对肾上腺皮质功能不全,特别是由于外伤、手术或严重疾病时的应激状态、由负氮产生的蛋白异化作用、电解质不平衡、由于糖尿病加重、包括高血糖、糖尿引起的葡萄糖代谢改变、可逆性但难以逆转的骨质疏松、自发性骨折髋部和肱骨无菌性坏死、消化性溃疡的致活与并发症,包括穿孔和出血、加剧或掩盖感染、血压升高、惊厥、瘀点和紫癜、月经失调,包括闭经、出血点或长期出血、失眠、精神紊乱,特别是异常的欣快、神经过敏、偶尔需摘除的后囊下白内障、眼内张力增加,由视神经乳头水肿引起的颅内压增加(假脑瘤)、胰腺炎、坏死性脉管炎、头皮毛发变细、儿童生长阻抑、面部红斑、皮肤过敏性反应、溃疡性食管炎、多汗、眩晕、虚弱、肌病、头痛、眼球突出。
皮下或皮肤萎缩,注射后潮红,无菌脓肿,色素沉着过高过低都与注射的皮质激素有关。
当副作用发生时,它们往往是可逆的,停用激素副作用就消失。
除胃肠道溃疡和出血有潜在性增加外,与用皮质激素治疗的脑外伤引起的脑水肿病人的有关副作用当疗程相对较短时不经常发生。发生在不同甾体药物治疗的颅内疾病病人身上的胃肠出血的危险似乎比用皮质激素治疗的有所增加。对颅内术后病人的追溯调查表明:男性胃肠出血的发生机率多于女性;昏迷病人最有可能出现胃肠出血,而且如果需要呼吸器,出血可能性增加;皮质激素治疗增加出血危险,其危险可能与剂量大小和治疗时间长短成比例。
〔剂量与用法〕
1.休克(见警告) 现代医学实践中,治疗严重休克往往使用大剂量肾上腺皮质甾类药物(药理学的)。建议48h内每4~6h静脉给药10~20min以上,30mg/kg甲泼尼龙琥珀酸钠。
2.免疫抑制 静脉给予大剂量甲泼尼龙琥珀酸钠用于预防或治疗器官移植排斥(特别是肾移植)的免疫抑制方案。剂量范围0.5mg~2g,每24h、48h静脉给药(见注意事项)。
虽然用大剂量,短期使用皮质激素治疗有关的副作用并不多见,但可能发生消化性溃疡,可采用预防性抗酸剂治疗。
3.大脑水肿 建议治疗由脑瘤引起的水肿用下列剂量表。
表25 甲泼尼龙用于由脑瘤引起的水肿的治疗剂量
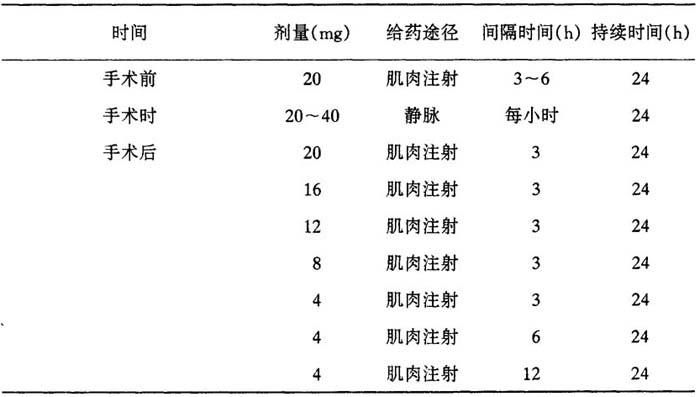
表26 甲泼尼龙用于由脑瘤引起的水肿的治疗剂量
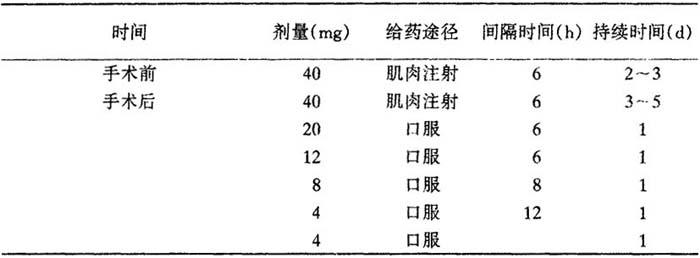
目的在于10d后停止治疗。
治疗由脑外伤引起的水肿时,建议用40~125mg甲泼尼龙琥珀酸钠,4~6h一次静脉或肌肉注射,4~7d。病人病情一稳定就停止治疗。
〔其他适应证〕
其他适应证根据临床治疗情况,首剂10~40mg不等。严重的急症处理可能需要较大剂量。首剂通常应静脉给药60s以上。以后的剂量可静脉给药根据病人反应、临床情况需要间隔给药。皮质激素治疗是一种辅助常规治疗,不是取代常规治疗。
婴儿和儿童剂量可以减少,但根据病人的年龄或体重,更应根据病人的严重程度和反应来控制。不少于0.5mg/(kg·d)。
用药数日后,必须减少剂量可逐渐停药。如果慢性病出现自发性缓解,治疗要停止。长期治疗时,要定期进行常规化验,如尿分析,饭后2h血糖,测量血压,测量体重,X线胸部透视。有溃疡史消化不良的病人要做上部胃肠X线检查。
无菌甲泼尼龙琥珀酸钠可以静脉注射、肌肉注射或静脉滴注给药,急症首剂宜用静脉注射。静脉(或肌肉)注射要按说明配制溶液。要配制静脉滴注溶液,首先按说明配制注射液,然后向注射液加入规定量的50%葡萄糖水溶液,等渗盐溶液或加5%葡萄糖等渗盐溶液。
给20~40mg做保留灌肠,2周或2周以上,每周连续滴3~7次已被证明是对某些溃疡性结肠炎病人的有用的辅助治疗。根据病人结肠粘膜受累程度,许多病人用40mg甲泼尼龙琥珀酸钠以1~10液量盎司(18.4~284m1)水给药可以得到控制。当然,应该着手使用其它可以接受的治疗措施。
混合玻瓶使用说明:
1.除去防尘帽,把瓶塞转动1/4,按压,使稀释液进入下面空间。
2.轻轻摇动,使生成溶液。48h内使用。
3.用适当的杀菌剂把瓶塞上部消毒。
4.通过瓶塞中心框插入针头,至恰好可见针尖部位,将瓶倒转,吸出一次剂量。
〔包装〕
灭菌甲泼尼龙琥珀酸钠以下列包装供应:
40mg混合瓶
125mg混合瓶
500mg带稀释剂玻瓶
1000mg带稀释剂玻瓶
〔生产厂家〕
比利时皮尔斯普强药厂
【外文释文】:
(methylprednisolone sodium succinate)
Description
Methylprednisolone sodium succinate,occurs as a white,or nearly white,odorless hygroscopic,amorphous solid.It is very soluble in water and in alcohol;it is insoluble in chloroform and is very slightly soluble in acetone.
This sodium succinate ester of Medrol(methylprednisolone),the 6-methyl derivative of predinisolone,is so extremely soluble in water that it may be administered in a small volume of diluent and is especially well suited for intravenous use in situations in which high blood levels of methylprednisolone are required rapidly.Solu Medrol(methylprednisolone sodium succinate)is available as:
40 mg Mix-O-Vial:each ml(when mixed)contains methylprednisolone sodium succinate equiv.to 40 mg methylprednisolone;also sodium biphosphate,dried sodium phosphate,lactose,benzyl alcohol,and water for injection.
125 mg Mix-O-Vial:each 2 ml(when mixed)contains methylprednisolone sodium succinate equiv.to 125 mg methylprednisolone;also sodium biphosphate,dried sodium phosphate,benzyl alcohol,and water for injection.
500 mg Vial:each 8 ml(when mixed as directed)contains methylprednisolone sodium succinate equiv.to 500 mg methylprednisolone,also sodium biphosphate,dried sodium phosphate,benzyl alcohol,and water for injection.
1 000 mg Vial:each 16 ml(when mixed as directed)contains methylprednisolone sodium succinate equiv.to 1 000 mg methylprednisolone,also sodium biphosphate,dried sodium phosphate,benzyl alcohol,and water for injection.
2 g Vial:each 32 ml(when mixed as directed)contains methylprednisolone sodium succinate equiv.to 2 g methylprednisolone,also sodium biphosphate,dried sodium phosphate,benzyl alcohol,and water for injection.
When necessary the pH of the 125 mg,500 mg,100 mg and 2 g formulas was adjusted with sodium hydroxide and/or hydrochloric acid.
IMPORTANT-Only the accompanying diluent is to be used when dissolving the Solu-Medrol in vials.
Use within 48 hours after mixing.
When reconstituted as directed,pH’s of the solutions range from 7-8 and the tonicities are,for the 40 mg per ml solution,0.50 osmolar,for the 125 mg per 2 ml,500 mg per 8 ml,1000 mg per 16 ml and 2 g per 32 ml solutions 0.40 osmolar(isotonic saline-0.28 osmolar).
Actions
Methylprednisolone is a potent anti-inflammatory steroid synthesized in the Research Laboratories of The Upjohn Company.It has a greater anti-inflammatory potency than prednisolone and even less tendency than prednisolone to induce sodium and water retention.
Methylprednisolone sodium succinate has the same metabolic and anti-inflammatory actions as methylprednisolone.When given parenterally and in equimolar quantities,the two compounds are equivalent in biologic activity.The relative potency of Solu-Medrol(methylprednisolone sodium succinate)and hydrocortisone sodium succinate,as indicated by depression of eosinophil count,following intravenous administration,is at least four to one.This is in good agreement with the relative oral potency of methylprednisolone and hydrocortisone.
Indications
Intravenous administration is indicated in situations in which a rapid and intense hormonal effect is required.These include the following:
1.Endocrine disorders
Acute adrenocortical insufficiency(hydrocortisone or cortisone is the drug of choice;mineralocorticoid supplementation may be necessary,particularly when synthetic analogues are used).
2.Collagen diseases
During an exacerbation or as maintenance therapy in selected cases of systemic lupus erythematosus.
3.Dermatologic diseases
(1)Pemphigus
(2)Severe erythema multiforme(Stevens-Johnson syndrome)
(3)Exfoliative dermatitis
(4)Generalized neurodermatitis
4.Allergic states
Control of severe or incapacitating allergic conditions intreatable to adequate trials of conventional treatment in:
(1)Bronchial asthma
(2)Contact dermatitis
(3)Serum sickness
(4)Drug hypersensitivity reactions
To control severe or incapaci tating allergic conditions intreatable to adequate trials of conventional treatment in angio-oedema and urticaria and as an adjunct to epinephrine in anaphylaxis.
Gastrointestinal diseases
To tide the patient over a critical period of disease in ulcerative colitis(systemic therapy or as an enema or drip).
Cerebral oedema
In cerebral oedema corticosteroids are being used to reduce or prevent cerebral oedema associated with brain tumours(primary or metastatic),head injuries,and intracranial surgery.
In patients withoedema due to tumour,tapering the dose of corticoid appears to be important in order to avoid a rebound increase in intracranial pressure.If brain swelling does occur as the dose is reduced(intracranial bleeding having been ruled out),restart larger and more frequent doses parenterally.Patients with certain malignancies may need to remain on oral corticoid therapy(e.g.Medrol 12-36 mg per day)for months or even life.Similar or higher doses may be helpful to control oedema during radiation therapy.In oedema due to brain trauma,therapy should be initiated as early as possible.
·Organ transplantation
To prevent or treat rejection of organ transplants.
·Acute rheumatic fever
·Severe shock
Haemorrhagic-Traumatic-Surgical.
Adjunctive use of intravenous Solu-Medrol may aid in achieving haemodynamic restoration.Corticoid therapy should not replace standard methods of combating shock,but present evidence indicates that concurrent use of large doses of corticoids with other measures may improve survival rates.
·As adjunctive therapy in croup
Some investigators have found corticoids to be of value in the treatment of moderately severe and severe“croup”(acute laryngotracheobronchitis)when administered to infants and children in conjunction with other accepted therapeutic measures.At the present time,however,there are no data concerning the effect of corticoids in the acute epiglottic form of croup due to H.influenzae.There is no evidence that the incidence of tracheostomies is decreasedby the administration of Solu-Medrol.The recommended dose is 40 mg intramuscularly,administered as early in the attack as possible.This therapy has been well tolerated.Transient bradycardia has been reported to occur occasionally in patients treated with this agent.
·In oesophageal burns due to ingestion of caustic,corticosteroid therapy has been associated with a lower incidence of structure formation and decreased morbidity.In order to be effective,corticoids must be started within 48 hours of the time the burn is sustained.A rapidacting steroid such as Solu-Medrol may be given in conjunction with supportive fluid and antibiotic therapy as initial treatment oesophagoscopy the drug may be stopped in patients who have no burns.Treatment of those.After with oesophageal damage should be continued with Depc-Medrol(methylprednisolone acetate)or oral Medrol,if tolerated,antibiotics and bougienage where required.
Contraindications
Except when used for short-term of emergency therapy as in acute sensitivity reactions,Solu-Medrol(methylprednisolone sodium succinate)like any corticoid is usually considered to be absolutely contraindicated in patients with herpes simplex keratitis,acute psychosis and in patients with latent healed or active tuberculosis.However concurrent administration of corticosteroids with anti-tuberculous agents may be life saving in certain cases of pulmonary or meningeal tuberculosis.Corticosteroids should not be given unless the tubercle bacilli have been shown to be sensitive to the antituberculous agents being employed.The following conditions are considered to be relative contraindications:active or latent peptic ulcer,Cushing’s syndrome,diverticulitis,fresh intestinal anastomoses,osteoporosis,renal insufficiency,thromboembolic tendencies,psychotic tendencies,diabetes mellitus,hypertension,local or systemic infections including vaccinia and varicella,as well as fungal diseases and other exanthematous diseases.
Pregnancy is a relative contraindication to corticoid therapy,particularly during the first trimester because of the observation of foetal abnormalities in experimental animals.If it is necessary to give corticosteroids during pregnancy,the newborn infant should be watched closely for signs of hypoadrenalism and appropriate therapy instituted if such signs are present.
If corticoids are employed in the above conditions,the risks should be weighed against possible benefits.
Warnings
Corticosteroids can cause elevation of blood pressure,salt and water retention,and increased excretion of potassium and calcium.In comparison with cortisone or hydrocortisone,synthetic corticosteroids have less mineralocorticoid effects.Dietary salt restriction and potassium supplementation may be necessary.
When corticoids are being used to treat cerebral oedema due to brain trauma,gastrointestinal bleeding may occur,and daily stool guaiac tests may be diagnostically helpful.In addition,there is the possibility that corticoid therapy may mask intracranal bleeding.
Data from a clinical study conducted to establish the efficacy of Solu-Medrol in septic shock,suggest that a higher mortality occurred in subsets of patients who entered the study with elevated serum creatinine levels or who developed a secondary infection after therapy began.
Precautions
Solu-Medrol(methylprednisolone sodium succinate)should be given only with full knowledge of the characteristic activity of,and the varied responses to,adrenocortical hormones.
There have been a few reports of cardiovascular collapse associated with the rapid intravenous administration of large doses of Solu-Medrol(>0.5g)in organ transplant recipients.The cause and relation to other medications(e.g.diuretics)is not known at this time,but physicians should be alert to this possibility.
Because of its inhibitory effect on fibroplasia,methylprednisolone may mask the signs of infection and enhance dissemination of the infecting organism.Hence,all patients receiving methylprednisolone should be watched for evidence of intercurrent infection.Should infection occur,it must be brought under control by use of appropriate antibacterial measures.
If possible,abrupt cessation of corticosteroid therapy should be avoided because of the danger of superimposed adrenocorticoid insufficiency on the infectious process.
Prolonged hormone therapy usually causes a reduction in the activity and size of the adrenal cortex.
Relative adrenocortical insufficiency upon discontinuation of therapy may be avoided by gradual reduction of dosage.However,a potentially critical degree of insufficiency may persist asymptomatically for some time even after gradual discontinuation of adrenocortical steroids.Therefore,if a patient is subjected to significant stress,such as surgery,trauma or severe illness while being treated or within one year(occasionally up to two years)after treatment has been terminated,hormone therapy should be augmented or reinstituted and continued for the duration of the stress and immediately following it.Since mineralocorticoid secretion may be impaired,salt and/or desoxycorticosterone should be administered conjunctively.It is preferable to use a soluble hormone preparation in the immediate preoperative and postoperative periods.
While a retardant effect on wound healing is seldom encountered,except in high doses,it should be a matter of consideration when Solu-Medrol is administered in conjunction with surgery.
Although unlikely with Solu-Medrol,average and large doses of corticosteroids can cause elevation of blood pressure,salt and water retention and increased potassium and calcium excretion.Dietary salt restriction and potassium supplementation may be necessary.Like other glucocorticoids,Solu-Medrol may aggravate diabetes mellitus so that higher insulin dosage may become necessary or manifestation of latent diabetes mellitus may be precipitated.The use of steroids in myasthenia gravis may aggravate myasthenia symptoms and should,therefore,be given either proper precautions.
Weakness of voluntary musculature has been reported following systemic administration of any of the anti-inflammatory steroids.In some instances this has been attributed to hypopotassaemia.Excessive loss of potassium,like excessive retention of sodium,is not likely to be induced by effective maintenance doses of methylpredinisolone.However,this effect should be kept in mind and periodic determinations of serum potassium performed in patients receiving any corticoid for prolonged periods.
Current investigations indicate that weakness of the voluntary musculature in patients receiving corticoids may occur in the presence of normal serum potassium levels and may be due to a disturbance in muscle metabolism.Patients who have developed severe myopathv have received corticoids in substantial dosage for prolonged periods.Presently available data indicate that severe myopathy complicating steroid therapy occurs more frequently in those patients receiving steroids containing the 9-alpha-fluoro configuration.In some instances improvenment in steroid-induced myopathy has been noted following withdrawal of the fluorinated steroid and institution of therapy with cortisone,hydrocortisone or a predni-steroid such as Medrol(methylprednisolone).The incidence of muscle weakness or wasting in patients receiving methylprednisolone appears to be low.
Retardation of linear growth has been noted in children receiving corticoids for 6 months or longer,the retardation being roughly proportional to the dose.Following cessation of therapy,the growth rate may be accelerated.For this reason,the growth of children receiving prolonged steroid therapy should be observed carefully.If growth has been retarded,the dose should be reduced sufficiently to permit recovery before epiphyseal closure.Rarely anaphylactoid reactions have been reported of following parenteral Solu-Medrol therapy.Physicians using the drug should be prepared to deal with such a possibility.
Since spontaneous remission of some diseases,such as rheumatoid arthritis,may occur during pregnancy,every effort should be made to avoid hormone treatment in pregnancy.Long-term adrenocorticoid therapy may evoke a rise in hyperacidity or peptic ulcer,therefore,as a prophylactic measure an ulcer regimen and the administration of an antacid are highly recommended.X-Rays should be taken in peptic ulcer patients complaining of gastric distress,and,whether or not changes are noted,an ulcer regimen is recommended.
Injection into the deltoid muscle should be avoided because of a high incidence of subcutaneous atrophy.
Continued supervision of the patient after cessation of Solu-Medrol therapy is essential,since there may be a sudden reappearance of severe manifestations of the disease for whichthe patient was treated.
In cerebral oedema due to brain trauma,prophylactic peptic ulcer therapy(i.e.,antacids,etc.)should be considered.
Adverse reactions
Adverse reactions associated with use of corticoids include:Cushing’s syndrome,moon facies,supraclavicular fat pads,hirsutism,striae and acne,relative adrenocortical insufficiency particularly in time of stress due to trauma,surgery or severe illness,protein catabolism with negative nitrogen balance,electrolyte imbalance,alteration of glucose metabolism with aggravation of diabetes mellitus including hyperglycaemia and glycosuria,osteoporosis reversible only with difficulty,spontaneous fractures,aseptic necrosis of the hip and humerus,activation and complication of peptic ulcer including perforation and haemorrhage,aggravation or masking of infection,increased blood pressure,convulsions,petechiae and purpura,menstrual irregularities including amenorrhoea,spotting or prolonged bleeding,insomnia,psychic disturbances especially abnormal euphoria,nervousness,posterior subcapsular cataracts occasionally requiring extraction,increased intraocular tension,increased intracranial pressure with papilledema(pseudotumour cerebri),pancreatitis,necrotizing angiitis,thinning of scalp hair,suppression of growth in children,facial erythema,allergic reactions involving the skin,ulcerative oesophagitis,sweating,vertigo,weakness,myopathy,headache,exophthalmos.
Subcutaneous and cutaneous atrophy,postinjection flare,sterile abscess,hyper-and hypopigmentation have been associated with injected corticoids.
When adverse reactions occur,they are usually reversible and disappear when the hormone is discontinued.
Except for a potential increase in gastrointestinal ulceration and bleeding,side efects associated with corticoid therapy in patients with cerebral oedema due to brain trauma are infrequent when the treatment course is relatively short.The risk of GI bleeding which may occur in patients with intracranial disease not receiving steroids,appears to increase with corticoid therapy.A retrospective survey of patients who had undergone intracranial surgery indicates that,1)men develop GI bleeding more frequently than women,2)the comatose patient is the most likely candidate to have GI bleeding and this chance is increased if a respirator is required,and 3)corticoid therapy increases the risk of bleeding.Risk may be proportional to the magnitude of the dose and duration of therapy.
Dosage and administration
Shock(see Warnings)
In treating severe shock there is a tendency in current medical practice to use massive(pharmacologic)doses of corticosteroids.The recommended dose of Solu-Medrol sterile powder is 30 mg per kg of methylprednisolone sodium succinate,given over a 10-to 20-minute period iv every 4-6 hours for 48 hours.
Immunosuppression
Large doses of intravenously administered Solu-Medrol(methylprednisolone sodium succinate)are being used in immuno-suppressive regimens to prevent or treat rejection of organ transplants(particularly renal).Doses ranging from 0.5-2.0 g may be administered intravenously every 24-48 hours(see Precautions).
Although adverse effects associated with high dose short-term corticoid therapy are uncommon,peptic ulceration may occur.Prophylactic antacid therapy may be indicated.
Cerebral oedema
The following are suggested dosage schedules for oedema due to brain tumour:
Table 25
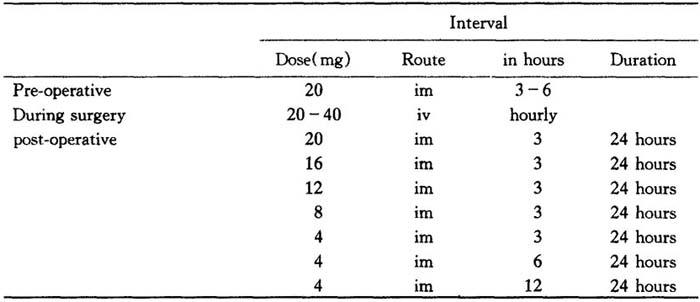
Table 26

Aim to discontinue therapy after a total of 10 days.
In treating edema due to brain trauma,Solu-Medrol,40 to 125 mg every four to six hours iv or im for four to seven days is suggested.Discontinue therapy as soon as the patient’s condition has stabilized.
Other indications
In other indications initial dosage will vary from 10 to 40 mg depending on the clinical problem being treated.The larger doses may be required for short-term management of severe acute conditions.The initial dose usually should be given intravenously over a period of 60 seconds.Subsequent doses may be given intravenously or at intervals dictated by the patient’s respones and clinical condition,corticoid therapy is an adjunct to,and not replacement for conventional therapy.
Dosage may be reduced for infants and children but should be governed more by the severit of the condition and response of the patient than by age or size.It should be not less than 0.5 mg per kg every 24 hours.
Dosage must be decreased or discontinued gradually when the drug has been administered for more than a few days.If a period of spontaneous remission occurs in a chronic condition,treatment should be discontinued.Routine laboratory studies,such as urinalysis,twohour postprandial blood sugar,determination of blood pressure and body weight,and a chest X-ray should be made at regular intervals during prolonged therapy.Upper GI X-rays are desirable in patients with an ulcer history or significant dyspepsia.
Sterile Solu-Medrol may be administered by intravenous or intramuscular injection or by intravenous infusion,the preferred method for initial emergency use being intravenous iniection.To administer by intravenous(or intramuscular)injection,prepare solution as directed.
To prepare solutions for intravenous infusion,first prepare the solution for injection as directed.This solution may then be added to indicated amounts of 5%dextrose in water,isotonic saline solution or 5%dextrose in isotonie saline solution.
Doses of 20 to 40 mg administered as retention enemas or by continuous drip three to seven times weekly for periods of two or more weeks have been shown to be a useful adiunct in the treatment of some patients with ulcerative colitis.Many patients can be controlled with 40 mg of Solu-Medrol administered in from 1 to 10 fluidounces of water depending on the degree of involvement of the inflamed colonic mucosa.Other accepted therapeutic measures should,of course,be instituted.
Directions for using the Mix-0-Vial
1.Remove protective cap,give the plunger-stopper a quarter turn and press to force diluent into the lower compartment.
2.Gently agitate to effect solution.Use solution within 48 hours.
3.Sterilize top of plunger-stopper with a suitable germicide.
4.Insert needle squarely through center or plunger-stopper until tip is just visible.Invert vial and withdraw dose.
How supplied
Sterile Solu-Medrol(methylprednisolone sodium succinate)is available in the following packages.
40 mg Mix-O-Vial
125 mg Mix-O-Vial
500 mg Vial with diluent
1000 mg Vial with diluent
2 gram Vial with diluent
Manufacturer
Upjo hn S.a.Puurs-Belgium