38.胰高血糖素
出处:按学科分类—医药、卫生 军事医学科学出版社《临床常用进口药物手册》第202页(5267字)
【中文释文】:
胰高血糖素有两种含量规格:
1mg:单一剂量包装,内有一支装有1mg(1U)盐酸胰高血糖素冻干粉的小玻璃瓶。这种包装也可以带一个1ml注射用水的小玻璃瓶或1支供一次性使用的消毒注射器和用作稀释剂的注射用水。
10mg:多剂量包装,内有一支装有10mg(10U)盐酸胰高血糖素冻干粉的小玻璃瓶。这种包装也可带有一个装10ml稀释剂的小玻璃瓶。
〔适应证〕
胰高血糖素用于因注射胰岛素或糖尿病病人口服抗糖尿病药以及精神病病人停用胰岛素休克疗法后发生的严重低血糖反应的治疗。胰高血糖素也可作为附加剂被推荐用于胃肠道放射造影或内窥镜检查以及治疗β-阻滞剂中毒。
〔用法及用量〕
成人和儿童:
1.治疗严重的低血糖反应 将冻干的胰高血糖素溶解于附加的稀释剂中,皮下、肌肉或静脉注射胰高血糖素0.5~1mg(0.5~1U)。如病人在10~15min内不发生反应,即可重复用此剂量或静脉注射葡萄糖。当病人有反应时,要口服糖类以恢复肝糖原和预防继发性低血糖。
2.停止胰岛素休克疗法 将冻干的胰高血糖素溶解于附加的稀释剂中。为终止胰岛素昏迷可皮下、肌肉或静脉注射胰高血糖素0.5~2mg(0.5~2U)。如病人在10~15min内未苏醒过来,再重复用此剂量或静脉注射葡萄糖。一旦病人苏醒,要尽快口服给药。处于深度昏迷状态时,可将葡萄糖与胰高血糖素同时静脉注射。
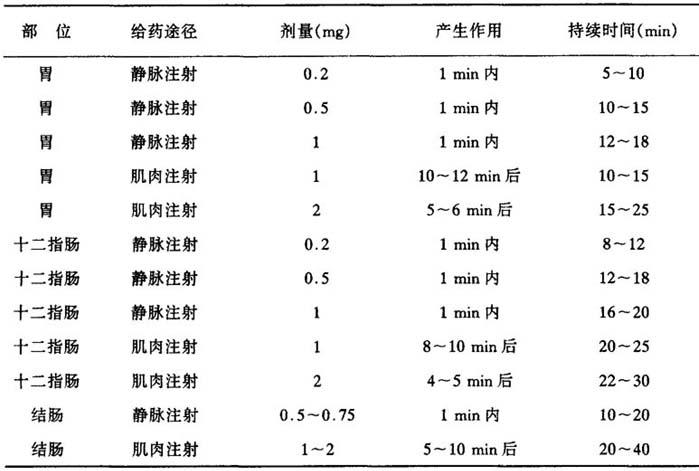
3.胃肠道放射造影与内窥镜检查 将冻干的胰高血糖素溶于附加的稀释剂中。剂量0.2~2mg(0.2~2U),并应按下表对各个脏器进行检查。下表显示剂量、给药途径及对胃、十二指肠和结肠产生低渗作用之间的关系。
表14 不同剂量给药途径用胰高血糖素对部分器官产生低渗作用之间的关系
4.β-阻滞剂中毒 将冻干的胰高血糖素溶于附加的稀释剂中。静脉注射10mg,此量可重复使用或随后以2mg/h速度作静脉滴注。
〔禁忌证〕
禁忌用胰高血糖素治疗嗜铬细胞瘤、胰岛瘤和高血糖瘤。
〔注意事项〕
如果对糖尿病病人应用胰高血糖素作为附加剂进行胃肠道放射造影和内窥镜检查时必须仔细观察。因为胰高血糖素是多肽,从理论上来讲,有过敏的可能性。
在药液中出现小纤维或固体颗粒时,任何时候都禁止使用。
〔贮藏〕
在包装上注有失效期。
1mg包装:药液应在配制后立即使用。
10mg包装:药液应在配制后立即使用。但在4℃时能贮存1周。如药液不洁不要使用。
〔生产厂家〕
丹麦哥本哈根 诺沃工业公司
(附本品别名:高血糖素,胰高糖素,升血糖,果开恩,果开康,Glukagon)
【外文释文】:
Glucagon Novo is available in two strengths:
1mg:a single-dose pack consisting of a glass vial containing glucagon hydrochloride 1 mg(1 I.U.),freeze-dried.The package may also contain a vial with 1 ml water for injection or a sterilised disposable syringe with water for injection as diluent.
10 mg:a multiple dose pack consisting of a glass vial containing glucagon hydrochloride 10 mg(10 I.U.),freeze-dried.The package may also contain a vial with 10 ml diluent.
Indications
Glucagon Novo is indicated in the treatment of severe hypoglycaemic reactions which may occur following insulin injections or oral antidiabetics in the management of diabetic patients,and in terminating insulin shock therapy in psychistric patients.Glucagon Novo is also recommended as an adjunct for use in examinations of the gastrointestinal tract by radiography or endosocopy and for treatment of β-blockers poisoning.
Dosage and Administration
Adults and children:
1.Treatment of severe hypoglycaemic reactions.Dissolve the freeze-dried glucagon in the accompanying diluent.Inject 0.5-1 mg(0.5-1 I.U.)of Glucagon Novo subcutaneously,intramuscularly or intravenously.If the patient does not respond within 10-15 minutes,the dose may be repeated or intravenous glucose should be given.When the patient responds,administer orally carbohydrate to restore the liver glycogen and prevent secondary hypoglycaemla.
2.Termination of insulin shock terapy.
Dissolve the freeze-dried glucagon in the accompanying diluent.For the termination of insulin coma,give 0.5-2mg(0.5-2 I.U.)of Glucagon Novo by subcutaneous,intramuscular or intravenous injection.If the patent does not awaken within 10-15 minutes,the dose may be repeated or intravenous glucose should be given.Upon awaking,the patient should be fed orally,as soon as possibe.In very deep states of coma,intravenous glucose may be given concurrently with Glucagon Novo.
3.Radiography and endoscopy of the gastrointestinal tract.
Dissolve the freeze-dired glucagon in the accompanying diluent.The dosage required ranges from 0.2mg(0.2 I.U.)to 2mg(2 I.U.),and should be adjusted to the individual examination according to the table below,which shows the connection between dose,route of administration,and onset of the hypotonic effect for stomach,duodenum and colon.
Table 14

4.β-blocker poisoning.
Dissolve the freeze-dried Glucagon in the accompanying diluent.Administer 10 mg by intravenous injection.The dose may be repeated or followed by an intravenousinfusion of 2mg/h.
Contraindications
Glucagon Novo is contraindicated in phaeochromocytoma,insulinoma and glucagonoma.
Precautions
Caution must be observed if Glucagon Novo is used in diabetic patients as an adjunct in radiography or endoscopy of the gastro-intestinal tract.Since glucagon is a polypeptide,there is a theoretical possibility of hypersensitivity.
The pressence of fibril formation or solid paticles in the solutions is a contraindication to its use at any time.
Storage
The date of expiry is stated on the pack.
1 mg pack:The solution should be prepared immediately prior to use.
10 mg pack:The solution should be prepared immediately prior to use,but can be stored at 4℃ for up to one week.Do not use solution unless clear.
Manufacturer
Novo Industri A/S Denmark.