54.密钙息
出处:按学科分类—医药、卫生 军事医学科学出版社《临床常用进口药物手册》第297页(17953字)
【中文释文】:
(鲑鱼降钙素)用于调节矿物质的平衡和骨代谢。
〔成分〕
密钙息的活性成分主要是合成的鲑鱼降钙素。它的生物活性以国际单位(U)表示。一个国际单位相当于0.2μg纯肽的药量。
密钙息的注射或滴注剂型为
安瓿(1ml) 含有50U/ml
安瓿(1ml) 含有100U/ml
小瓶*(2ml) 含有200U/ml
和供鼻内使用的剂型为
喷雾瓶装,足供14次使用用量,每次50U
喷雾瓶装,足供14次使用用量,每次100U
喷雾瓶装,足供14次使用用量,每次200U
*小瓶中含有的溶液量足供注射4次,每次0.5ml,如少量丢失,那么就可能达不到理论上的剂量。
〔特性〕
降钙素是与钙代谢调节有关的一种激素。在维持骨量方面它通过骨和钙平衡的作用来影响甲状旁腺素的作用。在患有骨吸收和形成的疾病中,密钙息显着地减少骨钙的丢失,诸如骨质疏松症、变形性骨病和恶性骨质溶解症。抑制破骨细胞活性和刺激成骨细胞的形成和活性。密钙息抑制溶骨作用,从而使异常增高的血清钙降低。另外,它通过减少肾小管的再摄取而增加尿钙、磷和钠的排泌,然而,血清钙不会降低到正常范围以下。
密钙息具有止痛作用,特别是伴有骨痛的疾病。这种止痛作用可能是作用于中枢神经系统,因为在中枢神经系统的某些区域发现有与鲑鱼降钙素特异性结合的部位。
降钙素能减少胃液和胰腺外分泌腺的分泌,但不影响胃肠蠕动性,由于这些特性,所以密钙息已显示有利于急性胰腺炎的内科治疗。
所有降钙素都含有一个32个氨基酸的单链,它的氨基酸排列因物种不同而有差异。由于鲑鱼降钙素对受体的结合部位和亲和力比哺乳类(包括合成的人降钙素)的降钙素更大,所以密钙息在临床上的作用更强、更持久。
〔药代动力学〕
1.注射给药 密钙息肌肉和皮下注射后,绝对生物利用度大约70%,1h内达到最大的血浆浓度。消除半衰期70~90min。鲑鱼降钙素95%是通过肾脏排泌,2%以药物的原型排泄。表观分布容积0.15~0.3l/kg,蛋白结合率为30%~40%。
2.鼻内给药 各研究者使用不同的方法所获得的生物利用度的数据差异甚大。正如其它多肽激素情况一样,鲑鱼降钙素的血浆浓度是不能预测治疗反应的。相反,骨代谢适当的指标测定,诸如碱性磷酸酶和尿羟脯氨酸排泌,已证明与剂量有关。且可作为生物活性的可靠结果,而且应该被用来评估临床疗效。按照生物活性观点,密钙息喷鼻剂的生物活性大约是肌肉注射或皮下注射药物的一半。
〔适应证〕
1.骨质疏松症 早期和晚期的绝经后骨质疏松症;老年性骨质疏松症;继发性骨质疏松症,例如:皮质类固醇治疗或缺乏活动。为了防止骨质进行性丢失,应根据个体的需要适量地摄入钙和维生素D。
2.伴有骨质溶解和(或)骨质减少的骨痛 Paget骨病(变形性骨炎),特别是伴有下列情况的病人)。骨痛;神经并发症;骨周转增加,表现在血清碱性磷酸酶增高和尿羟脯氨酸排泌增加;骨病变进行性蔓延;不完全或反复骨折。
3.由下列情况引起的高钙血症和高钙危象 继发于乳房癌、肺或肾癌、骨髓瘤和其它恶性疾病的肿瘤性骨溶解。甲状旁腺功能亢进,缺乏活动或维生素D中毒。
必须对高钙血症危象的紧急情况进行急诊治疗,并以慢性状态进行长期治疗,直至对基本疾病进行有效的特殊治疗。
4.神经营养不良症或Sudeck病 由各种病因和易患因素所致,诸如创伤后痛性骨质疏松症、交感神经反射不良症、肩一臂综合征、灼痛和药物引起的神经病变。
5.急性胰腺炎
〔剂量和用法〕
密钙息可以通过鼻内,皮下,肌肉或静脉途径给药。
下表概括了各种适应证的用药的推荐途径。
表18 密钙息针对不同适应证的推荐用药途径
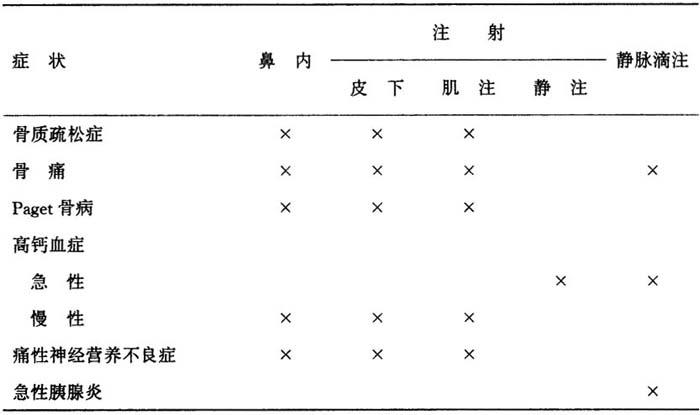
用4种推荐途径,不管局部或全身都有良好耐受性。
备注:小瓶所提供的溶液,能用于皮下或肌肉注射和用于连续不断的静脉滴注,但不适合于静脉推注,因为含有石炭酸(5mg/ml)作为防腐剂。
首次使用喷鼻之前,反复按压启动器以便启动排气泵直至释放均匀细小的气雾(详见说明书)。一旦使用,喷雾瓶应贮藏在室温下,并且在一个月内用完。如果喷雾器阻塞,可以通过强力的按压启动器来解除,请不要使用尖锐的物体。因为这会损伤喷雾器。
1.骨质疏松症
注射剂:50U/d或隔日100U/d,皮下注射或肌肉注射,取决于疾病的严重程度。
喷鼻剂:100U/d或每日,或隔日200U,一次或分次给药,取决于病人的反应。
2.伴有骨质溶解和(或)骨质减少的骨痛
视个体的需要而调整剂量。
注射剂:100~200U/d,置于生理盐水中缓慢静脉滴注或分次皮下注射或肌肉注射,直至达到满意反应。
喷鼻剂:200~400U/d,200U可以一次性给药;当需要大剂量时,应分次给药。
无论是注射或喷鼻给药,可以治疗几天,直至完全发挥药物的止痛作用。为了能长期治疗,通常应减少初始的日剂量和(或)延长给药间期。
3.变形性骨病
注射剂:每日或隔日100U,皮下注射或肌肉注射。
喷鼻剂:200U/d,一次或分次给药。某些病例,开始治疗时,分次给予400U是必要的。
无论使用何种方式给药,应连续至少3个月,必要时可更长些,剂量视病人的需要进行调整。
备注:在变形性骨病和其它一些骨高血钙性疾病中,密钙息的治疗至少几个月至几年时间。治疗能使血清碱性磷酸酶显着下降,并明显地减少了尿羟脯氨酸的排泌,常至正常值。然而,极少数病例,初期下降后再次增高,医师必须根据临床表现作出判断是否应该中止治疗和何时再恢复治疗。中止治疗后一或几个月可以再次发生骨代谢紊乱,对此必须重新应用一个疗程的密钙息治疗。
4.高钙血症
〔高钙血症危象的紧急处理〕
静脉滴注:给药是最有效的方式,应优先应用于紧急或其它严重疾病的处理。
注射剂:5~10U/(kg·d-1)置于500ml生理盐水中静脉滴注,持续6h,或分成2~4次缓慢静脉注射(注:小瓶内含有的溶液能用于静脉滴注,但一定不能用于静脉推注)。
1.慢性高钙血症的长期治疗
注射剂:5~10U/(kg·d-1),一次或分二次皮下注射或肌肉注射。治疗应根据病人的临床和生物化学反应进行调整,如果注射的密钙息剂量超过2ml,应优先肌肉注射和采取多个部位注射。
喷鼻剂:200~400U/d。200U可以一次性给药;当需要大剂量时,应分次给药。
2.痛性神经营养不良症早期诊断是必要的,一旦确认,就应开始治疗。
注射剂:100U/d,皮下或肌肉注射,连续2~4周,根据临床情况,可以进一步给予隔日100U达6周。
喷鼻剂:在2~4周期间,每日一次给药200U,根据临床情况进一步可以隔日给药200U达6周。
3.急性胰腺炎
注射剂:密钙息是保守治疗常用的一个佐剂。对此病,给予300U置于生理盐水中,静脉滴注24h,连续用药6d。
备注:自己皮下注射给药的病人必须接受医护人员正确的指导。
长期治疗的病人可以出现降钙素的抗体,然而,通常不影响临床疗效。长期治疗有时所见到的脱逸现象可能是由于结合部位饱和,显然与抗体的产生无关。在治疗中断之后,对密钙息的治疗反应可以恢复。
〔禁忌证〕
对密钙息过敏。(见副作用)
〔注意事项〕
鲑鱼降钙素不能通过动物胎盘,然而妊娠的妇女不宜使用密钙息。哺乳期不主张使用本药治疗,因为鲑鱼降钙素可以通过乳汁。
由于缺乏在儿童中长期使用本药的足够的资料,所以除非医师认为有长期治疗的指征,一般治疗期不要超过数周。
使用密钙息喷鼻剂的慢性鼻炎病人应定期医疗监查,因为鼻粘膜炎症时,可以增加药物的吸收。
由于鲑鱼降钙素是一中多肽,所以也可能出现过敏反应。对有过敏史病人,用药前应进行皮试。
密钙息应放置在儿童拿不到之处。
〔药物间的相互作用〕
尚无。
〔副作用〕
可以出现恶心、呕吐、头晕、轻度的面部潮红伴发热感。这些副反应与剂量有关,静脉注射比肌肉注射或皮下注射给药更常见,罕见的多尿和寒战已有报告。这些反应常常自发性地消退,仅在极少数的病例,需暂时性减少剂量。
在罕见的病例中,给予密钙息导致过敏反应,包括注射部位的局部反应或全身性皮肤反应。据报道个别的过敏反应可导致心动过速、低血压和虚脱。
鼻内途径给予密钙息时,副作用比较少见。
〔过量〕
迄今尚未报道过因过量引起的严重副反应。治疗视症状而定。
〔贮藏〕
为了长期保存,密钙息安瓿、小瓶和喷雾瓶应置放在2~8℃。一旦使用,小瓶和喷雾瓶可以置放在常温下,仅供使用4周。
〔使用说明〕
1.取下胶盖。
2.(仅用于第一次使用)如图(见说明书)所示一般握住喷鼻瓶向下按压直至出现“咔嗒”声,总共如此按压3次,动作完成时指示器显示绿色,说明喷鼻瓶已准备好可应用了。
3.将喷鼻瓶口放入一侧鼻孔按压一次,使药剂喷出。动作完成时,指示器上会显示“1”字,表示已给药一次。
4.如果医师要求您每次喷二次,则在另侧鼻孔如3的方法再多喷一次。
5.放加胶盖。
〔生产厂家〕
瑞士巴塞尔山道士制药公司
(附本品别名:降钙素,Calcitonin,Calcimar,Calcitare,Cibacalcin,Staporos)
【外文释文】:
Composition
One ampoule(1 ml)contains:Salmon calcitonin 100 U or 50 U(1 U=0.2 μg of the pure peptide).One bottle of nasal spray solution 50 contains at least 14 metered doses of 50 U salmon calcitionin.
One bottle of nasal spray solution 100 contains at least 14 metered doses of 100 U salmon calcitonin.
Properties/Actions
Calcitonin is one of the hormones involved in the regulation of calcium metabolism,inhibiting the action of parathyroid hormone.It considerably reduces the removal of calcium from bone in high-bone turn over disorders such as osteoporosis,Paget’s disease,algoneurodystrophy(Sudeck’s disease)and malingant osteolysis,its effect in postmenopausal osteoporosis being much more pronounced in the axial skeleton than in the extremities and in highthan in low-bone-turnover disease.It inhibits osteoclast activity while appearing to stimulate osteoblast formation and activity.Calcitonin also inhibits osteolysis,thus lowering pathologically raised serum calcium levels,as well as increasing urinary excretion of calcium,phosphorus and sodium by reducing tubular reabsorption,serum calcium does not fall below the normal range,however.
Calcitonin inhibits the secretory activity of the stomach and pancreas but without affecting gastrointestinal motility.
There is evidence that Miacalcic exerts an analgesic effect in some patients with painful bone disorders.While all calcitonins are structurally similar,having 32 aminoacids in a single chain of varying sequence.depending on the species,salmon calcitonin possesses greater affinity for its receptor binding sites(which have also been identified in certain areas of the CNS),giving it superior clinical efficacy and a longer duration of action than synthetic mammalian(including human)calcitonins.
Pharmacokinetics
Injectable solution
The absolute bioavailability of Miacalcic following intramuscular or subcutaneous injection is approximately 70%,its peak plasma concentration is attained within one hour and it has a half-life of elimination of 70-90 minutes.
Up to 95% of the dose is excreted via the kidneys,2% as unchanged drug.The apparent volume of distribution is 0.15-0.3 l/kg and protein binding 30-40%.
Intranasal solution
Reported bioavailability data vary considerably.As with other polypeptide hormones,blood level is not a good guide to therapeutic response,but a reliable indication of bioactivity may be obtained by measuring bone turnover indices such as seum alkaline phosphatase and urinary hydroxyproline,such methods should therefore be used to assess clinical efficacy.The bioactivity of the nasal spray is approximately half that of the injectable solution(by im or scadministration).
Indications/Uses
Substantiated uses
Osteoporosis
·Early and advanced postmenopausal osteoporosis where conventional oestrogen/calcium combination therapy is contraindicated or not possible for some other reason.
To prevent progressive loss of bone mass patients receiving Miacalcic must be given calcium and vitamin D supplementation appropriate to individual requirements.
Paget’s disease of bone(osteitis deformans)
Hypercalcaemia and hypercalcaemic crisis due to:
·Excessive osteolysis due to malignancy with bone metastases in breast,lung or kidney carcinoma,myeloma and other malignancies.
·Hyperparathyroidism,immobilization or vitamin-D intoxication,for both acute and chronic cases.
Algoneurodystrophy or Sudeck’s disease
Associated wiht various causes and predisposing factors such as posttraumatic painful osteoporosis,sympathetic reflex dystrophy,shoulder-hand syndrome,causalgia,iatrogenic neurotrophic disturbances.
Dosage and administration
Miacalcic may be given by the intranasal,subcutaneous,intramuscular or intravenous route.The recommended routes of administration for the various indications are summarized in the following table:
Table 18
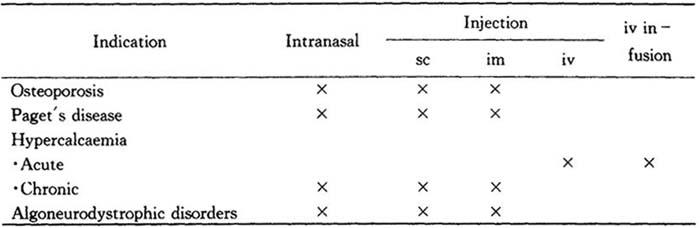
The drug is well tolerated both locally and systemically by all four routes at the recommended dose levels.
Postmenopausal osteoporosis
The lowest effective dose is not known and recommended dose levels are currently as follows:
Injectable form:50-100 U daily or 100 U every second day sc or im,depending on the severity of the disease.
Nasal spray:100-200 U(1 or 2 metered doses of the 100 U solution or 2-4 metered doses of the 100 U solution or 2-4 metered doses of the 50 U solution)daily or 200 U every second day depending on severity,if necessary in several divided doses.
Paget’s disease
Injectable form:100 U daily by sc or im injection.Subcutaneous injection is well tolerated and may be self-administered by the patient after appropriate instruction by the doctor ornurse.Injection every second day in sufficient in some cases,while a regimen of only 50 Udaily may suffice,particularly following an improvement in clinical signs and symptoms,Onthe other hand the dose may be increased to 200 U daily if necessary.
Nasal spray:200 U(2 metered doses of the 100 U solution or 4 metered doses of the 50U solution)daily in two divided doses,although 200 U twice daily may be required initiallyin a few cases.Dose reduction may also be attempted after a time in patients using the intranasal form.
Hypercalcaemia
Emergency management of hypercalcaemic crisis
Intravenous drip infusion is the most effective method of administration and should always be used in emergencies or severe cases.
Injectable form:5-10 U per kg bodyweight in 500 ml physiological saline daily by ivdrip infusion over at least six hours or by slow,iv injection in 2-4 divided doses spread over the day.The patient must be rehydrated.Where necessary emergency management should be followed by specific treatment for the underlying disease.
Long-term management of chronic hypercalcaemic states
Injectable form:5-10 U per kg bodyweight daily,depending on the patient’s clinical and biochemical response,by sc or im injection in a single dose or two divided doses.If the volume to be injected exceeds 2 ml,the im route should be used.Injections should be given at different sites.
Nasal spray:200-400 U daily in several divided doses.
Algoneurodystrophic disorders
Early diagnosis is important and treatment with Miacalcic should start as soon as it is confirmed.
Injectable form:100 U daily sc or im for 2-4 weeks,then 100 U three times a week for up to 6 weeks,depending on the patient’s response.
Nasal spray:200 U daily in 2-4 divided doses for 2-4 weeks,then 200 U three times a week for up to 6 weeks,depending on response.
N.B.
In Paget’s disease and other chronic conditions involving increased bone turnover treatment is required for several months or even years.Serum alkaline phosphatase and urinary hydroxyproline excretion fall significantly,often to normal levels,and pain is partially or completely relieved.It occasionally happens,however,that alkaline phosphatase and hydroxyproline levels rise again following the initial fall,in which case the physician must decide on the basis of the clinical picture whether treatment should be continued.
If Miacalcic is withdrawn,abnormal bone metabolism may recur after one or more months,necessitating,its restoration.Although antibodies may develop in some patients undergoing long-term therpy with calcitonin,its clinical efficacy is not normaly affected.The loss of efficacy(escape phenomenon)that sometimes occurs with long-term use of the drug is probably due to saturation of the binding sites and does not paaear to be related in any way to the binding sites and does not appear to be related in any way to the development of antibodies.Responsiveness to calcitonin returns following a break in treatment.
Long-term use of the nasal spray has not been assqciated with reports of pathological changes involoving the nasal mucosa.
Restrictions on use
Contraindications
Hypersensitivity to Miacalcic.
1.Precautions
As a polypeptide,calcitonin very occasionally gives rise to local or generalized hypersensitivity reactions.In patients with a history of such reactions consideration should therefore be given to performance of a skin test before instituting treatment with the drug,If signs of hypersensitivity occur that are clearly drug related treatment should be discontinued.
2.Use in children
As there is insufficient experience of the long-term use of Miacalcic in children,treatment should not be given for periods of more than a few weeks unless the physician considers that prolonged treatment is indicated on compelling medical grounds.
Chronic rhinitis may increase the bioavailability of intranasally administered calcitonin and patients with this condition should be carefully monitored if they are using the nasal spray.
3.Pregnancy and lactation
Reproduction studies in animals do not suggest that there is any risk of foetal damage,but this has not been confirmed by controlled trials in pregnant women.The drug does not cross the placental barrier in animals.Breastfeeding duting treatment with Miacalcic is not recommended as there is evidence that it passes into breastmilk.
Adverse reactions
Nausea and vomiting are common,while dizziness and flushing may also occur.These effects,which are dose dependent,are more frequent after i.v.than after i.m.or s.c.administration.There have also been isolated reports of polyuria and chila.All these reactions normally stop occurring spontaneously,though tempoary dose reduction is very occasionally necessary.
In rare cases calcitonin injection may give rise to hypersensitivity reactions,these usually take the form of local effects at the injection sith or generalized skin reactions,but anaphylactic-type reactions resulting in tachycardia,hypotension and syncope have also been reported in a few cases.
Side effects are less common with the intranasal than with the injectable form.
Interactions
There are no known interactions with other drugs,although systematic studies have not been performed.
Overdosage
There have been no reports of severe adverse reactions due to overdosage.
Other items of information
Instructions for use
Nasal spray:before the device is used for the first time,the pump must be primed by depressing the actuator three times until the colour showing in the notch in the bottom rim of the plunger is green.If the nozzle should become blocked at any time,try to expel the blockage by firm depression of the actuator.Do not attempt to unblock by means of a needle orother sharp object as this may damage the spray mechanism.
Storage
Ampoules and unopened nasal spray bottles should be stored in a refrigerator(2-8℃).Once opened the nasal spray bottle must be kept at room temperature,it should be kept in the upright position and used for a maximum of 4 weeks.
Like all drugs,Miacalcic should be kept out of reach of children.
Shelf-life
The drug should not be used after the expiry date(=EXP)printed on the pack.
Manufacturer
Sandoz Pharma,Ltd.,Basle Switzerland